Only Device Approved to Deliver First Disease-Modifying Treatment for AADC Deficiency in the United States
SOLANA BEACH, CA / ACCESSWIRE / November 13, 2024 / ClearPoint Neuro, Inc. (Nasdaq:CLPT) (the "Company"), a global therapy-enabling platform company providing navigation and delivery to the brain, today announced the U.S. Food and Drug Administration (FDA) has granted marketing authorization for the SmartFlow Neuro Cannula using the De Novo pathway. The SmartFlow Neuro Cannula is intended for intraputaminal administration of PTC Therapeutics' gene therapy KEBILIDI™ (eladocagene exuparvovec-tneq) for the treatment of aromatic L-amino acid decarboxylase (AADC) deficiency. This signifies the first-ever FDA marketing authorization of a device used to deliver gene therapy directly to regions of interest within the brain.
"Today is one of the most important strategic milestones in the history of our company," commented Joe Burnett, President and CEO at ClearPoint Neuro. "FDA's granting of this De Novo classification is the culmination of years of disciplined co-development, creative problem solving, and tireless efforts. We could not be prouder of the PTC and ClearPoint Teams and how we have worked together. Most importantly, we are thrilled and humbled that children with this terrible disease will now be treated with the first-ever neuro gene therapy available commercially in the United States."
"I am proud to have served as a PI in the trial supporting today's landmark approvals," stated Dr. Daniel Curry, Director, Functional Neurosurgery and Epilepsy Surgery at Texas Children's Hospital and Professor, Neurosurgery and Surgery at Baylor College of Medicine. "I have witnessed first-hand the positive impact these treatments have had on children with AADC Deficiency. Neurosurgeons are leading the way in this promising field to address the underlying genetic cause, and not just the symptoms, of devastating neurological disorders by delivering gene therapy directly to targets in the brain through dedicated platforms."
"Today's De Novo approval will give confidence to all our current and future biopharma partners that ClearPoint has the experience to help their cell and gene therapies cross the finish line," commented Jeremy Stigall, Chief Business Officer and Biologics and Drug Delivery Leader at ClearPoint Neuro. "We offer a complete medical device solution so that our pharma partners can concentrate on the development of their drug and trust us to handle all aspects related to delivery. We are effectively a medical device division that works on behalf of pharma partners, providing surgical strategy, benchtop testing, compatibility assessments, pre-clinical study management, clinical trial support, precise navigation, infusion monitoring software, and a routinely audited quality management system and manufacturing processes. Furthermore, we can customize devices for new routes of administration that leverage our unique intellectual property and experience with the FDA to co-develop products that are ideally suited to different cell and gene therapy suspensions and different targets in the brain and spine. More than anything, we offer our partners a head start, and now have proof that we can take these projects to commercial clearance."
"This pioneering treatment has the potential to truly transform the standard of care for children with this disorder and this platform holds promise for many other conditions," said Dr. Gerald A. Grant, The Allan H. Friedman Distinguished Professor of Neurosurgery, Chair of the Dept. of Neurosurgery at Duke University. "I feel so privileged to have been part of this clinical trial team and am hopeful that this innovation will open doors for treating many other conditions. The power of hope is so impactful for these children and their families."
About aromatic L-amino acid decarboxylase (AADC) deficiency
AADC deficiency is a fatal, rare genetic disorder that typically causes severe disability and suffering from the first months of life, affecting every aspect of life-physical, mental and behavioral. The suffering of children with AADC deficiency may be exacerbated by episodes of distressing seizure-like oculogyric crises causing the eyes to roll up in the head, frequent vomiting, behavioral problems, and difficulty sleeping.
The lives of affected children are severely impacted and shortened. Ongoing physical, occupational and speech therapy, and interventions, including surgery, also are often required to manage potentially life-threatening complications such as infections, severe feeding and breathing problems.
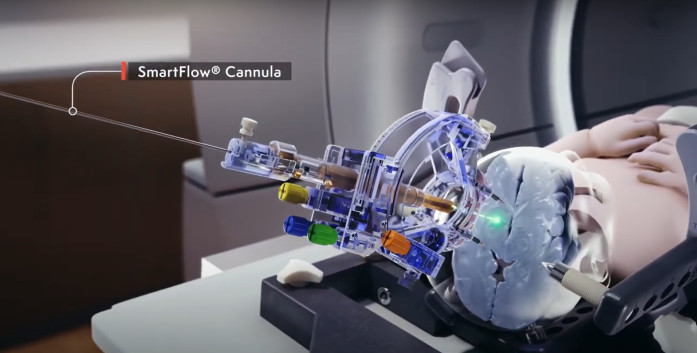
About the SmartFlow Neuro Cannula
With over 8,000 cannulas sold to date, SmartFlow is the only co-labeled device to gain approval by regulatory bodies in both the U.S. and EU for delivery of an approved gene therapy to the brain. The industry-leading cannula is used by many of ClearPoint Neuro's 50+ pharmaceutical, academic, and biotech partners to bypass the blood brain barrier and deliver therapeutics to regions of interest using Convection Enhanced Delivery (CED) under direct image guidance. The SmartFlow Neuro Cannula has received De Novo FDA approval for the intraputaminal administration of the gene therapy KEBILIDI™ (eladocagene exuparvovec-tneq) for the treatment of aromatic L-amino acid decarboxylase (AADC) deficiency. SmartFlow is also 510(k) cleared by the FDA for use in the United States for the aspiration of cerebrospinal fluid or injection of the chemotherapy drug Cytarabine into the ventricles. The cannula is CE marked to deliver cytarabine and other approved fluids into the brain or perform aspiration of CSF. SmartFlow is being utilized in approved clinical and preclinical studies for various research and drug trials.
About ClearPoint Neuro
ClearPoint Neuro is a device, cell, and gene therapy-enabling company offering precise navigation to the brain and spine. The Company uniquely provides both established clinical products as well as preclinical development services for controlled drug and device delivery. The Company's flagship product, the ClearPoint Neuro Navigation System, has FDA clearance and is CE-marked. ClearPoint Neuro is engaged with healthcare and research centers in North America, Europe, Asia, and South America. The Company is also partnered with the most innovative pharmaceutical/biotech companies, academic centers, and contract research organizations, providing solutions for direct CNS delivery of therapeutics in preclinical studies and clinical trials worldwide. To date, thousands of procedures have been performed and supported by the Company's field-based clinical specialist team, which offers support and services to our customers and partners worldwide. For more information, please visit www.clearpointneuro.com.
Forward-Looking Statements
This press release may contain forward-looking statements within the context of the federal securities laws, which may include the Company's expectations for the future performance, market, and revenue of its products and services. These forward-looking statements are based on management's current expectations and are subject to the risks inherent in the business, which may cause the Company's actual results to differ materially from those expressed in or implied by forward-looking statements. Particular uncertainties and risks include those relating to: global and political instability, supply chain disruptions, labor shortages, and macroeconomic and inflationary conditions; future revenue from sales of the Company's products and services; the Company's ability to market, commercialize and achieve broader market acceptance for new products and services offered by the Company; the ability of our biologics and drug delivery partners to achieve commercial success, including their use of the Company's products and services in their delivery of therapies; the Company's ability to maintain its current relationships with biologics and drug delivery partners or enter into new relationships with such partners; the Company's expectations, projections and estimates regarding expenses, future revenue, capital requirements, and the availability of and the need for additional financing; the Company's ability to obtain additional funding to support its research and development programs; the ability of the Company to manage the growth of its business; the Company's ability to attract and retain its key employees; and risks inherent in the research, development, and regulatory approval of new products. More detailed information on these and additional factors that could affect the Company's actual results are described in the "Risk Factors" section of the Company's Annual Report on Form 10-K for the year ended December 31, 2023, and the Company's Quarterly Report on Form 10-Q for the three months ended September 30, 2024, both of which have been filed with the Securities and Exchange Commission. The Company does not assume any obligation to update these forward-looking statements.
Contact Information
Jacqueline Keller
Vice President of Marketing
[email protected]
(888) 287-9109 ext. 4
Danilo D'Alessandro
Chief Financial Officer
[email protected]
(888) 287-9109 ext. 3
Related Video
https://www.youtube.com/watch?v=80MiTDxykl8
SOURCE: ClearPoint Neuro
View the original press release on accesswire.com