PR Newswire
MARLBOROUGH, Mass., Sept. 17, 2024
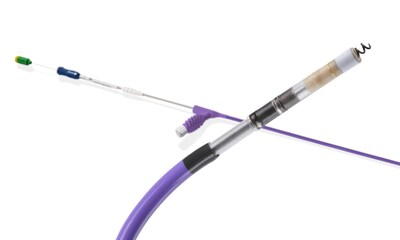
MARLBOROUGH, Mass., Sept. 17, 2024 /PRNewswire/ -- Boston Scientific Corporation (NYSE: BSX) has received U.S. Food and Drug Administration (FDA) approval to expand the indication for current-generation INGEVITY™+ Pacing Leads – thin wires placed inside the heart and connected to an implantable device – to include conduction system pacing (CSP) and sensing of the left bundle branch area (LBBA) when connected to a single- or dual-chamber pacemaker.
Pacing of the LBBA is an alternative to traditional right ventricular pacing for the treatment of symptomatic bradycardia, a condition in which the heart beats too slowly. Through this pacing approach, which uses the heart's natural electrical system, a lead is placed in the LBBA of the heart's conduction system. This technique may promote greater ventricular synchrony and reduce the long-term risk of heart failure associated with traditional right ventricular pacing.1
"This approval strengthens our broader initiative to provide physicians with LBBA-specific tools and educational resources, while reinforcing our commitment to developing safe and effective pacing technologies," said Scott Olson, senior vice president and president, Cardiac Rhythm Management and Diagnostics, Boston Scientific. "We believe the expanded indication for the INGEVITY+ Pacing Lead will enhance the implant experience for physicians and connect this technology to the growing number of patients who can benefit from LBBA pacing."
Clinical evidence submitted to the FDA to support the expanded indication included data from approximately 400 patients from the INSIGHT-LBBA study – an analysis of INGEVITY+ pacing leads that were previously implanted in the LBBA for anti-bradycardia pacing – and supplemented with bench testing and LATITUDE™ Programming System data.
"This expanded indication provides physicians using the INGEVITY+ Pacing Lead the flexibility to determine the most appropriate pacing strategy based on individual patient characteristics," said Kenneth Stein, M.D., senior vice president and global chief medical officer, Boston Scientific. "Data has demonstrated this lead to be safe and effective for LBBA pacing – a rapidly growing pacing technique – allowing us to provide a new therapeutic option on a proven lead that will further the quality of patient care."
The INGEVITY+ Pacing Lead is driven by a stylet during lead placement, which supports positioning the device into a desired location within the heart and allows for both continuous pacing and impedance monitoring – key features that can aid appropriate placement and fixation. The expanded indication follows the launch of the Boston Scientific CSP portfolio – inclusive of the OneLINK™ Splitter Cable, INGEVITY+ Helix Locking Tool and site-selective pacing delivery catheters – which is designed to support the safe and effective placement of the INGEVITY+ Pacing Lead in the LBBA. The INGEVITY+ lead received FDA approval in 2019 for use with pacemakers and defibrillators.
More information on the INGEVITY+ Pacing Lead is available here.
About Boston Scientific
Boston Scientific transforms lives through innovative medical technologies that improve the health of patients around the world. As a global medical technology leader for more than 40 years, we advance science for life by providing a broad range of high-performance solutions that address unmet patient needs and reduce the cost of healthcare. Our portfolio of devices and therapies helps physicians diagnose and treat complex cardiovascular, respiratory, digestive, oncological, neurological and urological diseases and conditions. Learn more at www.bostonscientific.com and connect on LinkedIn and X, formerly Twitter.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements may be identified by words like "anticipate," "expect," "project," "believe," "plan," "estimate," "intend" and similar words. These forward-looking statements are based on our beliefs, assumptions and estimates using information available to us at the time and are not intended to be guarantees of future events or performance. These forward-looking statements include, among other things, statements regarding our business plans and product performance and impact, and new and anticipated product approvals and launches. If our underlying assumptions turn out to be incorrect, or if certain risks or uncertainties materialize, actual results could vary materially from the expectations and projections expressed or implied by our forward-looking statements. These factors, in some cases, have affected and in the future (together with other factors) could affect our ability to implement our business strategy and may cause actual results to differ materially from those contemplated by the statements expressed in this press release. As a result, readers are cautioned not to place undue reliance on any of our forward-looking statements.
Factors that may cause such differences include, among other things: future economic, competitive, reimbursement and regulatory conditions; manufacturing, distribution and supply chain disruptions and cost increases; variations in outcomes of ongoing and future clinical trials and market studies; new product introductions; demographic trends; intellectual property; litigation; financial market conditions; and future business decisions made by us and our competitors. All of these factors are difficult or impossible to predict accurately and many of them are beyond our control. For a further list and description of these and other important risks and uncertainties that may affect our future operations, see Part I, Item 1A – Risk Factors in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission, which we may update in Part II, Item 1A – Risk Factors in Quarterly Reports on Form 10-Q we have filed or will file hereafter. We disclaim any intention or obligation to publicly update or revise any forward-looking statements to reflect any change in our expectations or in events, conditions or circumstances on which those expectations may be based, or that may affect the likelihood that actual results will differ from those contained in the forward-looking statements, except as required by law. This cautionary statement is applicable to all forward-looking statements contained in this document.
CONTACTS:
Steve Bailey
Media Relations
(651) 582-4343 (office)
[email protected]
Jon Monson
Investor Relations
(508) 683-5450
[email protected]
1 Sharma P et al. Clinical outcomes of left bundle branch area pacing compared to right ventricular pacing: Results from the Geisinger-Rush Conduction System Pacing Registry. Heart Rhythm. 2021; 19:3-11
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/boston-scientific-receives-fda-approval-for-expanded-indication-of-ingevity-pacing-leads-to-include-conduction-system-pacing-of-the-left-bundle-branch-area-302249916.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/boston-scientific-receives-fda-approval-for-expanded-indication-of-ingevity-pacing-leads-to-include-conduction-system-pacing-of-the-left-bundle-branch-area-302249916.html
SOURCE Boston Scientific Corporation